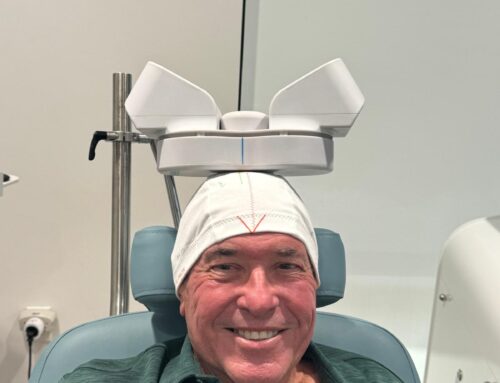
The Food and Drug Administration (FDA) in September cleared the SAINT Neuromodulation System for the treatment of refractory depression in adults. SAINT is an innovative form of transcranial magnetic stimulation (TMS) that combines MRI-guided selection of the targeted brain region with an accelerated stimulation regimen involving multiple short TMS sessions every day for five days.
“This is more than just clearance of another device. This clearance expands the way we can use TMS to treat depression,” Mark S. George, M.D., distinguished professor of psychiatry, radiology, and neuroscience at the Medical University of South Carolina, said in a media release from Magnus Medical Inc., the manufacturer of SAINT.
As George explained, older TMS protocols require six weeks or more of regularly scheduled stimulation sessions (three to five sessions a week). SAINT’s five-day protocol might expand options for patients who are hospitalized and/or present to the emergency room, as well as outpatients unable to commit to a six-week treatment regimen.
The clearance of SAINT was based off multiple clinical studies that showed rapid, robust, and sustained improvements in depression symptoms in adults who had received SAINT. One of these studies was published last October in The American Journal of Psychiatry. The trial included 29 adults with treatment-resistant depression who were assigned to either SAINT or sham stimulation. Participants who received SAINT experienced on average a 62% reduction in their Montgomery-Åsberg Depression Rating Scale (MADRS) scores following five days of stimulation compared with a 14% drop in MADRS scores in those receiving sham stimulation. These mood improvements were maintained over four weeks. At the four-week follow-up, 69% of the participants in the SAINT group had a treatment response (at least a 50% improvement in the MADRS score) and 46% met the criteria for remission (MADRS scores of less than or equal to 10).
The FDA clearance of SAINT was groundbreaking for many reasons, explained Nolan Williams, M.D., the director of the Stanford University Brain Stimulation Lab, which developed the SAINT protocol. In addition to being the first rapid-acting neuromodulation commercially available, Williams told Psychiatric News that SAINT is also the first to use functional MRI to map out an individual’s brain connectivity to identify the optimal anatomic region for stimulation.
“Because SAINT requires neuroimaging, this clearance marks the first official involvement of radiology in mental illness care,” he said. (PET and other imaging tools are used to aid in some psychiatric diagnoses, but this marks the first use of imaging to treat patients with mental illness, according to Williams.) Williams, who is also an assistant professor of psychiatry and behavioral sciences at Stanford, said the arrival of SAINT may usher in a new medical subspeciality of psychoradiology.
Zafiris Daskalakis, M.D., Ph.D., chair of psychiatry at the University of California, San Diego (UCSD), told Psychiatric News this clearance could be transformative for psychiatry, though larger and more clinically diverse studies are needed (for example trials with depressed individuals with a comorbid personality disorder or substance use disorder).
In an editorial accompanying the AJP study, Daskalakis and UCSD colleague Cory R. Weissman, M.D., wrote that SAINT needs more testing in patients with severe depression who would currently be candidates for electroconvulsive therapy [ECT], such as psychiatric inpatients or patients with emergent suicidality; the existing clinical studies enrolled outpatients with moderate depressive symptoms. “If SAINT proves as efficacious as ECT it would offer a true alternative to patients and physicians who are worried about ECT’s cognitive side effects,” he said.
“I think it’s also important to see studies where the novel innovations of SAINT [MRI targeting and multiple sessions per day] are separated,” said Daskalakis. “Are the effects additive, synergistic, or is one element doing all the lifting.” For example, if the MRI-based target selection is not essential, it might open up SAINT to clinics with limited MRI access and/or patients whose insurance will not cover the cost of imaging.
Williams thinks the MRI requirement for SAINT will not be a significant roadblock once clinics and payers see the long-term value of SAINT. He noted that the MRI assessment is only about 30 minutes and only needs to be done once per patient. “When you factor in the rapid and long-term benefits from finding that optimal location in a large group of patients, it becomes very cost effective,” he said.
According to Brett Wingeier, Ph.D., co-founder and CEO of Magnus Medical, the SAINT system (which will include the magnetic stimulator as well as all the tools needed to analyze the MRI scans of patients using the company’s cloud-based algorithm) will be available for purchase in 2023. Wingeier co-founded Magnus with Brandon Bentzley, M.D., Ph.D., a former member of the Stanford Brain Stimulation Lab. Williams is not employed by Magnus but is an advisor for the company.
Sources:
Zagorski, Nick. “FDA Clears Accelerated TMS Protocol for Depression.” Psychiatry Online, https://doi.org/10.1176/appi.pn.2022.10.10.40.